- The U.K. administered the first Covid-19 vaccines to the public on Tuesday, making it one of the first countries in the world to do so.
- The Pfizer-BioNTech shots gained emergency approval from the U.K. drug regulator last week.
- It will be given first to front-line health workers, nursing home workers and those over age 80.
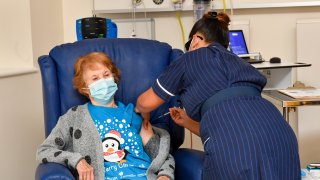
LONDON — The U.K. rolled out the first coronavirus vaccines to the public on Tuesday, making it the first country to inoculate people with a treatment that went through full testing.
Margaret Keenan, who turns 91 next week, made history as the world's first person to receive the Pfizer-BioNTech vaccine outside of trial conditions. The vaccine was approved by the U.K. drug regulator last week.
"I feel so privileged," she said. "It's the best early birthday present I could wish for because I can finally look forward to spending time with my family and friends in the New Year after being on my own for most of the year."
An 81-year-old man named William Shakespeare became the second person to get the vaccine. He was inoculated in Coventry. "No, no, not nervous at all," said Shakespeare, who lives in Warwickshire. "I'm very apprehensive about the side effects ... but there's a small chance of that."
Now, the vaccine will be given to front-line health workers, nursing home workers and those ages over 80, before it is given more widely among the U.K. population.
Money Report
On the eve of the vaccine being rolled out, U.K. Prime Minister Boris Johnson said it was a "huge step forward" in the fight against the pandemic. It will be the country's biggest vaccination drive ever.
Johnson's sentiment was echoed by NHS England's Chief Executive Simon Stevens, who said on Monday that it was a "decisive turning point in the battle against coronavirus."
British newspapers, meanwhile, hailed it "V Day" and "Vaxit" (a play on "Brexit" — the other big news in the U.K. this week).
'I am so proud'
The rollout comes at a crucial time for the country; the U.K. has the third-highest number of coronavirus cases in Europe, after France and Italy, with over 1.7 million confirmed infections, and more than 61,000 deaths, data from Johns Hopkins University shows.
Fifty hospitals have been chosen to act as vaccine "hubs" in the U.K. and these will act as the primary place where the inoculations are administered. Later, the vaccine will be rolled out to community health centers, such as doctors' surgeries, in order to facilitate a more general vaccination program, where the priority will depend on age and clinical need.
Croydon University Hospital in London was one of the first hospitals to receive batches of the vaccine this weekend.
"It's just incredible actually," Croydon Health Services' Chief Pharmacist Louise Coughlan told reporters.
"Obviously I can't hold them in my hands because they are minus 70 degrees, but to know that they are here and we are amongst the first in the country to actually receive the vaccine and therefore the first in the world is just amazing. I am so proud," she said.

The U.K. preordered 40 million doses of Pfizer and BioNTech's vaccine, which proved to be 95% effective at preventing Covid infection in late-stage clinical trials.
As it's a two-dose vaccine, the country has bought enough doses to vaccinate 20 million people. Pfizer's delivery of the vaccines will be staggered, with the total amount expected to have been delivered by the end of 2021.
Britain has also preordered other Covid-19 vaccines from AstraZeneca and Moderna, but these are yet to be granted approval.
From security issues to public confidence
Pfizer confirmed to CNBC that the U.K. will first receive around 800,000 shots from its manufacturing hub in Puurs, Belgium. However, there is secrecy around the actual delivery schedule. "We can't share any more on how or where it is coming into in the U.K. for security reasons," a company spokesperson told CNBC in a statement.
Aside from security issues, there are other logistical challenges posed by the vaccine's transportation and storage needs. The Pfizer-BioNTech vaccine can only be moved four times, has to be stored at minus 70 degrees Celsius (minus 94 degrees Fahrenheit), and once thawed, can only be stored at refrigerated temperatures for up to five days.
Another challenge for the government is public perception and participation in the vaccine program, amid the spread of anti-vaccine misinformation.
Last week, professor Jonathan Van-Tam, the U.K.'s deputy chief medical officer, warned that a "low uptake" of the vaccine could mean a continuation of coronavirus restrictions, and possible further lockdowns.
"Nobody wants lockdowns and to see the damage they do," he said during a government news conference. "But if you want that dream to come true (for life to go back to normal) as quickly as it can come true, then you have to take the vaccine when it is offered to you."
Anti-vaccination rhetoric
Surveys have indicated that the British public is generally supportive of receiving a Covid vaccine, and it will not be compulsory, but some are wary about the breakneck speed with which the vaccine underwent testing, and was granted approval by the U.K.'s Medicines and Healthcare products Regulatory Agency.

Andre Spicer, professor of organizational behavior at Cass Business School in London, told CNBC on Monday that building public confidence in the vaccine was a "huge issue" for governments.
"We know that anti-vaccination rhetoric is increasing and particularly during the Covid upswing," he told CNBC's "Street Signs," adding that the typical way governments respond is "to provide information saying it's safe, and you might also get leaders or influential people in a community to be seen taking the vaccine."
"But there's a lot of research to suggest that that does not tend to convince the most uncertain," he said. "With those people, you need to focus on people they actually know … like a family doctor or nurse," Spicer added.
Vaccine development and approval can often take many years, but the devastating spread of the coronavirus pandemic has seen scientists race to find a way to stop the virus. The vaccine front-runners also include those developed by Moderna and AstraZeneca, which have also reported that their shots were widely effective at preventing coronavirus infection in clinical trials.
The vaccine makers have insisted that no corners have been cut. The U.K. regulator was the first in the world to grant approval to Pfizer-BioNTech's vaccine last week, with its European counterpart expected to announce its conclusions about the Pfizer vaccine later this month, and the Moderna vaccine in early January.
The U.S. Food and Drug Administration is holding a meeting Thursday where it is expected to discuss an emergency use application for Pfizer and BioNTech's vaccine.
— CNBC's Marty Steinberg contributed to this report.






