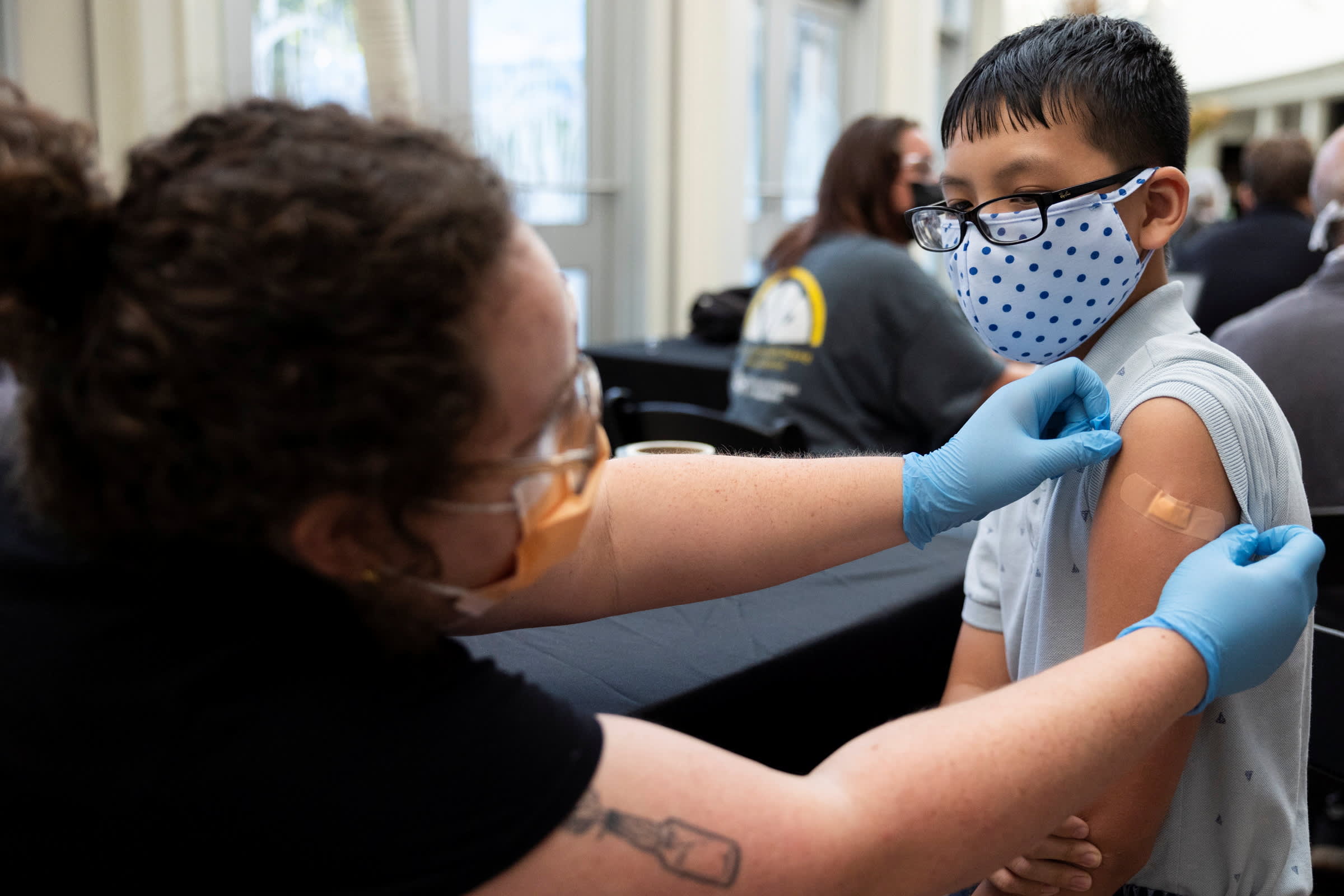
Pfizer says its COVID-19 vaccine works for children ages 5 to 11 and that it will seek U.S. authorization for this age group soon — a key step toward kids being vaccinated.
The company's vaccine is already available for anyone 12 and older. But with kids now back in school and the more highly transmissible delta variant causing a huge jump in pediatric infections, many parents are anxiously awaiting vaccinations for their younger children.
WATCH ANYTIME FOR FREE
>Stream NBC10 Boston news for free, 24/7, wherever you are. |
"Vaccination is the single most important intervention that we have available to help prevent COVID," said pediatrician Scott Hadland, who's also a parent and the chief of adolescent medicine at Massachusetts General Hospital. "Kids may not get serious illness to the same extent that older individuals do, but COVID will keep them out of school, it will keep them from learning, it will keep them from having a healthy development this school year, and so it is going to be a really key intervention in our schools."
For elementary school-aged kids, Pfizer tested a much lower dose — a third of the amount that's in each shot given now. Yet after their second dose, children between 5 and 11 developed coronavirus-fighting antibody levels just as strong as teenagers and young adults, Dr. Bill Gruber, a Pfizer senior vice president, told The Associated Press.
Get updates on what's happening in Boston to your inbox. Sign up for our >News Headlines newsletter.
The kid dosage also proved safe, with similar or fewer temporary side effects — such as sore arms, fever or achiness — that teens experience, Gruber said.
Hadland says health care professionals will have to reach parents who are hesitant.
"I think that's a real concern that there are going to be some parents that are going to be worried," he said. "And that's appropriate, that's your job as a parent, to make sure your child is getting a healthy intervention. I'm here to reassure that all of the data suggests that this is a healthy intervention."