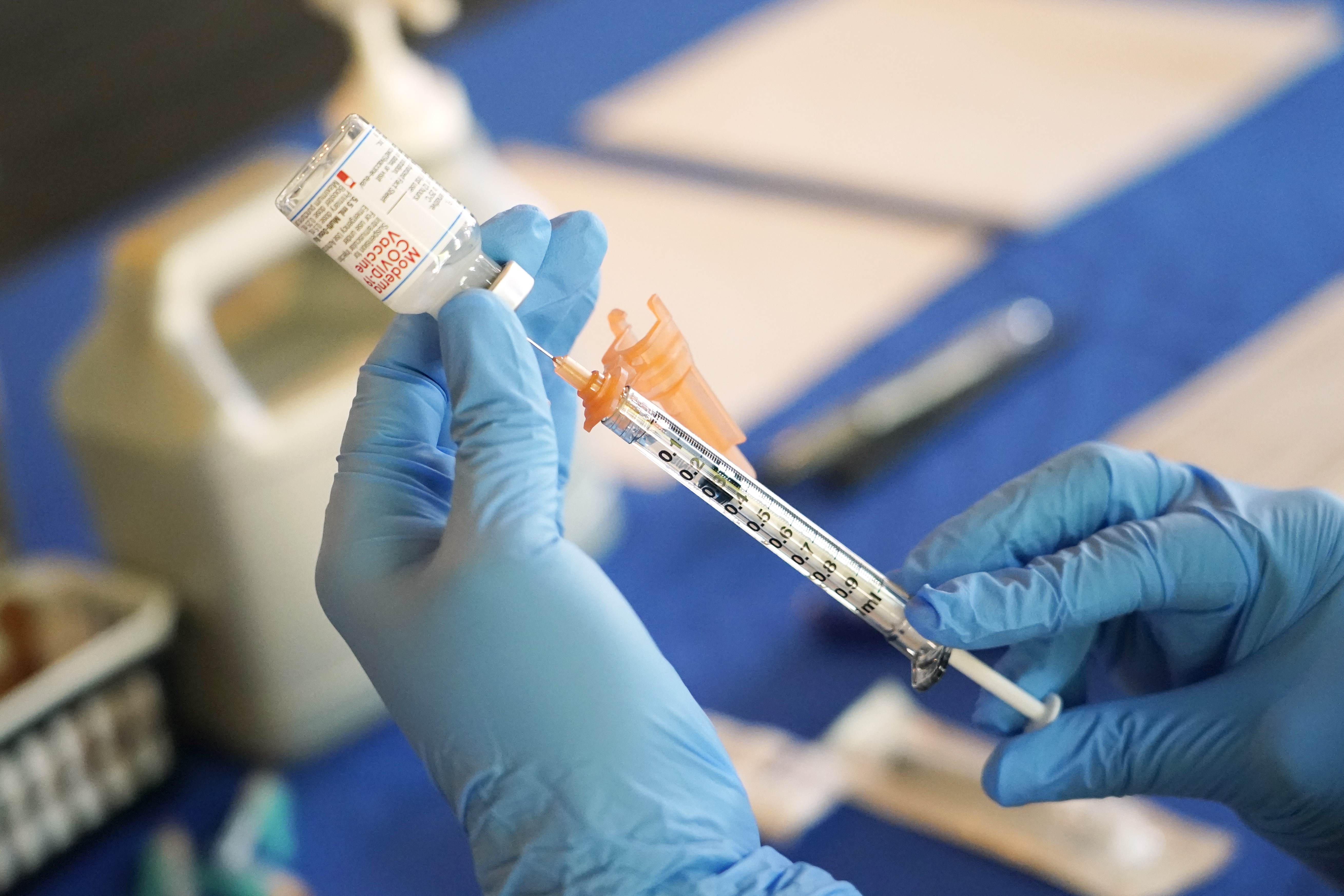
Scientists at Oxford University say their experimental coronavirus vaccine has been shown in an early trial to prompt a protective immune response in hundreds of people who got the shot.
British researchers first began testing the vaccine in April in about 1,000 people, half of whom got the experimental vaccine. Such early trials are usually designed only to evaluate safety, but in this case experts were also looking to see what kind of immune response was provoked.
Stream NBC10 Boston news for free, 24/7, wherever you are.
In research published Monday in the journal Lancet, scientists said that they found their experimental COVID-19 vaccine produced a dual immune response in people aged 18 to 55.
“We are seeing good immune response in almost everybody,” said Dr. Adrian Hill, director of the Jenner Institute at Oxford University. “What this vaccine does particularly well is trigger both arms of the immune system,” he said.
Get updates on what's happening in Boston to your inbox with our News Headlines newsletter.
Hill said that neutralizing antibodies are produced — molecules which are key to blocking infection. In addition, the vaccine also causes a reaction in the body's T-cells which help to fight off the coronavirus.
He said that larger trials evaluating the vaccine's effectiveness, involving about 10,000 people in the U.K. as well as participants in South Africa and Brazil are still underway. Another big trial is slated to start in the U.S. soon, aiming to enroll about 30,000 people.
How quickly scientists are able to determine the vaccine's effectiveness will depend largely on how much more transmission there is, but Hill estimated they might have sufficient data by the end of the year to decide if the vaccine should be adopted for mass vaccination campaigns.
Coronavirus Pandemic
Full coverage of the COVID-19 outbreak and how it impacts you
He said the vaccine seemed to produce a comparable level of antibodies to those produced by people who recovered from a COVID-19 infection and hoped that the T-cell response would provide extra protection.
“There's increasing evidence that having a T-cell response as well as antibodies could be very important in controlling COVID-19,” Hill said. He suggested the immune response might be boosted after a second dose; their trial tested two doses administered about four weeks apart.
Hill said Oxford's vaccine is designed to reduce disease and transmission. It uses a harmless virus — a chimpanzee cold virus, engineered so it can’t spread — to carry the coronavirus’ spike protein into the body, which should trigger an immune system response.
Hill said Oxford has partnered with drugmaker AstraZeneca to produce their vaccine globally, and that the company has already committed to making 2 billion doses.
“Even 2 billion doses may not be enough,” he said, underlining the importance of having multiple shots to combat the coronavirus.
“There was a hope that if we had a vaccine quickly enough, we could put out the pandemic,” Hill said, noting the continuing surge of infections globally. “I think its going to be very difficult to control this pandemic without a vaccine.”
Historically, it has taken a decade or more to develop a vaccine. And while some U.S. leaders say a vaccine for the new coronavirus could be ready as soon as late this year or early next, experts expect a longer timetable. But together they are counting on a never-before-seen marshaling of scientific, industry, philanthropic and government forces to drive the global effort.
In the U.S., a multibillion-dollar, public-private partnership called Operation Warp Speed is aiming to compress the vaccine development timeline by overlapping parts of the process normally conducted in sequence, including pre-clinical studies and clinical trials.
To be ready for quick distribution, the federal plan also calls for manufacturing the most promising vaccines before they get Food and Drug Administration approval. But with that comes the risk of discarding and absorbing the enormous expense of having produced vaccines that aren't deemed safe and effective by the FDA.
Numerous countries including Germany, France, the Netherlands, Italy and the U.K. have all signed deals to receive hundreds of millions of doses of the vaccine — which has not yet been licensed — with the first deliveries scheduled for the fall. British politicians have promised that if the shot proves effective, Britons will be the first to get it. British officials said Monday they had also signed a deal to buy 90 million doses of experimental COVID-19 vaccines being developed by the pharmaceutical giant Pfizer and others.
Last week, American researchers announced that the first COVID-19 vaccine tested there boosted people’s immune systems just as scientists had hoped and the shots will now enter the final phase of testing. That vaccine, developed by the National Institutes of Health and Moderna, produced the molecules key to blocking infection in volunteers who got it, at levels comparable to people who survived a COVID-19 infection.
Nearly two dozen potential vaccines are in various stages of human testing worldwide, with a handful entering necessary late-stage testing to prove effectiveness.
Vaccines will have to meet the unique needs of groups including the elderly, pregnant women, children and populations at high risk of severe illness from COVID-19, such as those with serious heart conditions, obesity and Type 2 diabetes. Such trials, experts say, can be challenging and take time – important considerations in managing expectations for speedy results.
There are roughly a half-dozen basic approaches to building a vaccine, plus variations on those, said Dr. Paul Offit, director of the Vaccine Education Center at the Children's Hospital of Philadelphia. These range from using an inactivated, or killed, version of a virus to launch the body's defenses – an approach pioneered by Jonas Salk in developing a polio vaccine – to cutting-edge and not-yet-proven strategies, such as using genetic material called messenger RNA to evoke an immune response.
Given that the virus that causes COVID-19 was identified just months ago, there's much to learn. For instance, it's not clear whether any particular vaccine-building strategy is superior, Offit said. And scientists will have to wait and see how long the effects of any new vaccine will endure, though previous work with coronaviruses suggests protection would last at least a few years.
A vaccine that's safe and works in at least 50% of people in clinical trials would be considered a viable candidate, said virologist Dr. Larry Corey, past president and director at Fred Hutchinson Cancer Research Center in Seattle, who heads the operations center for the COVID-19 Prevention Network. The network is a collaboration funded by the National Institute of Allergy and Infectious Diseases to optimize execution of the very large trials needed for each candidate vaccine identified as promising.
"Every new pathogen is a challenge," Offit said. "You learn as you go."